
Chemistry, 14.12.2021 21:50 ashleydawn6430
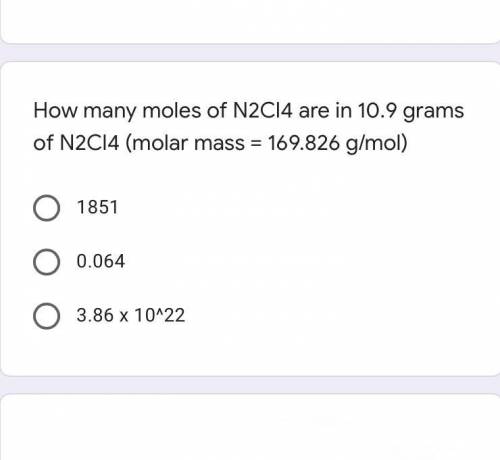
How many moles of N2Cl4 are in 10.9 grams of N2Cl4 (molar mass = 169.826 g/mol)
A. 1851
B. 0.064
C. 3.86 x 10^22

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:10, emilyplays474
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2


Chemistry, 22.06.2019 11:30, elijah1090
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 15:00, NatalieKnows
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
How many moles of N2Cl4 are in 10.9 grams of N2Cl4 (molar mass = 169.826 g/mol)
A. 1851
Questions in other subjects:


Physics, 20.10.2020 18:01

Mathematics, 20.10.2020 18:01

Business, 20.10.2020 18:01

Mathematics, 20.10.2020 18:01


Chemistry, 20.10.2020 18:01

Mathematics, 20.10.2020 18:01

English, 20.10.2020 18:01

Health, 20.10.2020 18:01




