Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, darrriannn7241
What is the correct lewis structure for chloroform chcl3
Answers: 1

Chemistry, 22.06.2019 19:40, powberier6979
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1


Chemistry, 23.06.2019 13:30, Catracho3619
32p and 31p are two isotopes of phosphorus. compare the number if subatomic particles that are present in the atoms of these isotopes.
Answers: 1
You know the right answer?
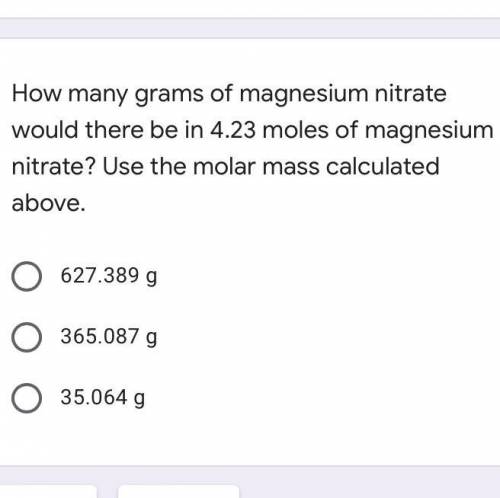
How many grams of magnesium nitrate would there be in 4.23 moles of magnesium nitrate?
A. 627.389...
Questions in other subjects:




Mathematics, 06.05.2020 03:24




Health, 06.05.2020 03:24

Mathematics, 06.05.2020 03:24