
Chemistry, 13.12.2021 07:10 heybrothwrlogan
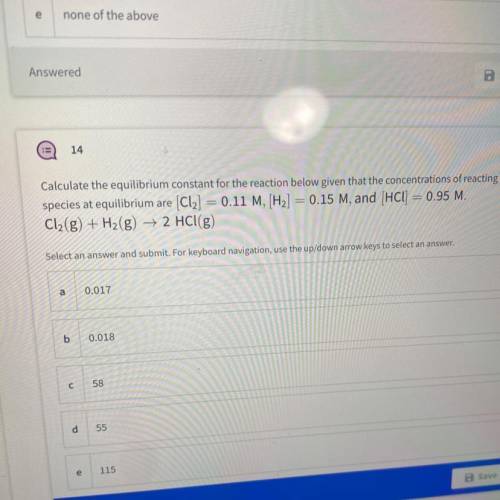
Calculate the equilibrium constant for the reaction below given that the concentrations of reacting
species at equilibrium are (Cl2] = 0.11 M, [H2] = 0.15 M, and (HCl) = 0.95 M.
Cl2(g) + H2(g) → 2 HCl(g)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, dontcareanyonemo
Select the correct answer. what is the nature of the se-cl bond in a molecule of selenium chloride (secl2) if the electronegativity value of selenium is 2.55 and that of chlorine is 3.16?
Answers: 3

Chemistry, 21.06.2019 21:30, gallegosarmanni
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 22.06.2019 12:00, Alexislol7908
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 12:40, whitethunder05
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2
You know the right answer?
Calculate the equilibrium constant for the reaction below given that the concentrations of reacting...
Questions in other subjects:



Mathematics, 09.07.2019 06:30

Physics, 09.07.2019 06:30

Social Studies, 09.07.2019 06:30


Mathematics, 09.07.2019 06:30

Social Studies, 09.07.2019 06:30

Social Studies, 09.07.2019 06:30

Mathematics, 09.07.2019 06:30



