
Chemistry, 10.12.2021 04:10 mylittleponeyfbrarit
Prediction: The Cucl, crystal will dissolve in water. If we write the solvation process as a chemical
equation, we can predict two possible outcomes:
a. Cuci
H2O Cu?* (aq) + 2 Cl(aq)
H2O Cu2+ (aq) + Cl2(9)
+ 9
.
b. Cuciz
In equation (a) Cl' ions remain dissolved in water whereas in equation (b) the chlorine molecule CI,
forms and leaves the solution as a gas. Which outcome do you expect (a or b)? Explain your reasoning.
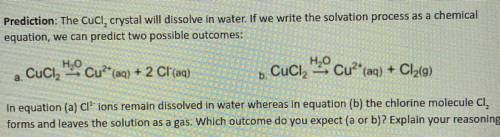

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:20, dgadam7495
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 02:30, bionicboy03120440
What is the mass of sodium in 3 moles of sodium chloride
Answers: 1

Chemistry, 22.06.2019 09:20, pandaman632
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1
You know the right answer?
Prediction: The Cucl, crystal will dissolve in water. If we write the solvation process as a chemica...
Questions in other subjects:

Chemistry, 14.01.2020 23:31




Biology, 14.01.2020 23:31


Mathematics, 14.01.2020 23:31



English, 14.01.2020 23:31



