
Chemistry, 09.12.2021 06:20 QueenNerdy889
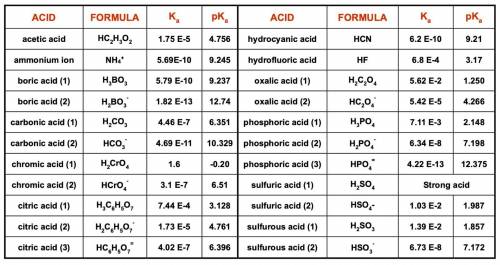
1. List the species present at equilibrium in a solution with the following
composition:
NH4Cl = 0.0200 mol/L NaOH = 0.0430 mol/L
H2SO4 = 0.0150 mol/L NaNO3 = 0.0100 mol/L
2. Write the n equations for n unknowns describing the equilibrium composition of
this system.
3. Make a spreadsheet and use Excel’s Solver function to determine the equilibrium
pH and concentrations of all species in this solution.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:00, bobbycisar1205
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 04:00, lucasrandall
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 11:40, tatemelliott
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3
You know the right answer?
1. List the species present at equilibrium in a solution with the following
composition:
Questions in other subjects:

Mathematics, 19.05.2021 05:40

Mathematics, 19.05.2021 05:40

Arts, 19.05.2021 05:40

Mathematics, 19.05.2021 05:40

Social Studies, 19.05.2021 05:40

History, 19.05.2021 05:40

English, 19.05.2021 05:40



History, 19.05.2021 05:40



