A substance (A) reacts to form another substance (B):
3A(g)
↔
2B(g)
The r...

A substance (A) reacts to form another substance (B):
3A(g)
↔
2B(g)
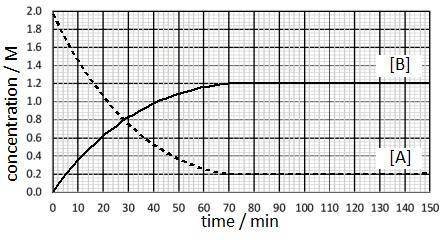
The reaction is run at a particular temperature with the concentrations of A and B monitored over time and plotted in the graph. At what time was equilibrium first reached and what is the approximate value of the equilibrium constant?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, angelinararr5783
Which answer lists the fundamental forces in order from strongest to weakest
Answers: 1

Chemistry, 22.06.2019 02:50, JuniperGalaxy
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 17:30, latezwardjr15
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 23:00, hailey5campbelp7d1c0
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2
You know the right answer?
Questions in other subjects:

Computers and Technology, 01.12.2021 03:20


History, 01.12.2021 03:20

Social Studies, 01.12.2021 03:20

English, 01.12.2021 03:20







