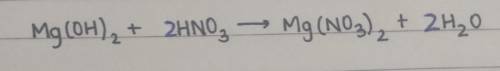Chemistry, 07.12.2021 23:20 Alexisgrab
When magnesium hydroxide reacts with nitric acid, it produces magnesium nitrate and water. Balance the following reaction:
__ Mg(OH)2 + __ HNO3 → __ Mg(NO3)2 + __ H2O
Please answer correctly. I will report you If you steal my points. I will also mark whoever answers first the brainliest (unless my computer doesn't let me)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:20, lex68259100
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 07:00, mayamabjishovrvq9
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 13:30, annanikherrera
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 23.06.2019 00:00, samangelzrose3576
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1
You know the right answer?
When magnesium hydroxide reacts with nitric acid, it produces magnesium nitrate and water. Balance t...
Questions in other subjects:



Mathematics, 24.02.2021 23:00


Mathematics, 24.02.2021 23:00


Mathematics, 24.02.2021 23:00


English, 24.02.2021 23:00

Chemistry, 24.02.2021 23:00