
Chemistry, 07.12.2021 19:40 isaiahcannon5709
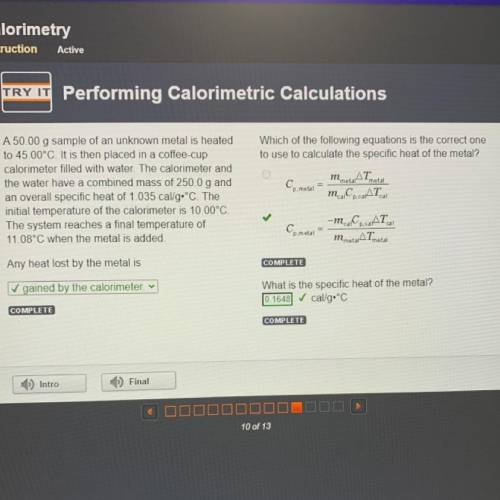
Which of the following equations is the correct one
to use to calculate the specific heat of the metal?
AT
Cp. metal
m. Co. AT
metal
A 50.00 g sample of an unknown metal is heated
to 45.00°C. It is then placed in a coffee-cup
calorimeter filled with water. The calorimeter and
the water have a combined mass of 250.0 g and
an overall specific heat of 1.035 cal/g•°C. The
initial temperature of the calorimeter is 10.00°C.
The system reaches a final temperature of
11.08°C when the metal is added.
metal
cal
Ce metal
-m. C.CAT
m metarATmetal
COMPLETE
Any heat lost by the metal is
✓ gained by the calorimeter v
What is the specific heat of the metal?
cal/g.°C
COMPLETE
DONE

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:00, tahjaybenloss16
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 02:00, hemolelekeakua
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
You know the right answer?
Which of the following equations is the correct one
to use to calculate the specific heat of the m...
Questions in other subjects:

Mathematics, 04.11.2020 22:00



History, 04.11.2020 22:00

Advanced Placement (AP), 04.11.2020 22:00

Chemistry, 04.11.2020 22:00

Spanish, 04.11.2020 22:00

Business, 04.11.2020 22:00

Social Studies, 04.11.2020 22:00

Mathematics, 04.11.2020 22:00




