
Chemistry, 06.12.2021 22:10 kyliegriffis
Consider the figure below. In the case of butane, diethyl ether, and water, the heat of vaporization is considerably larger than heat of fusion. Explain this phenomenon. Note that the nature of intermolecular interactions does not change through the phases because the molecules does not undergo chemical changes.
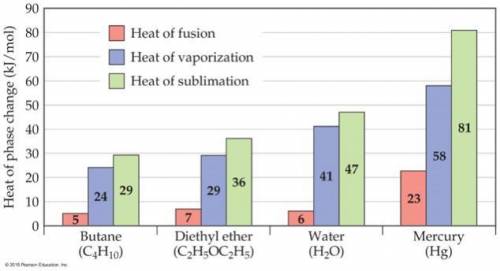

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, tifftifftiff5069
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1



Chemistry, 22.06.2019 14:00, ashlynneboogs0056
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
Consider the figure below. In the case of butane, diethyl ether, and water, the heat of vaporization...
Questions in other subjects:



Social Studies, 17.01.2020 01:31









