
Chemistry, 06.12.2021 06:00 phagonphillips4675
HELP
1. What is the pH at which a 10.0mM Os(NO3)2 will precipitate Os (OH)2 (s)? K3p of Os (OH)2 is 4.92 x 10^10 at 25.0C
7. Balance the following reaction under basic conditions: Al (s) + Cr2O72- (aq) -> Al3+ (aq) + Cr3+ (aq)
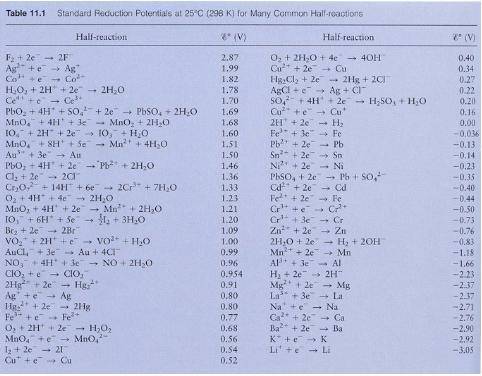
8. What is the ^o of the following balanced redox reaction (use the reduction potentials provided in Table 11.1
below)?
Fe3+ (aq) + Au (s) -> Fe (s) + Au3+ (aq)
9. What is G for the reaction below – calculate it from the ^o ?
Fe (s) + Au3+ (aq) -> Fe3+ (aq) + Au (s)

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:30, amandamiro05
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 23.06.2019 01:30, ayoismeisalex
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1
You know the right answer?
HELP
1. What is the pH at which a 10.0mM Os(NO3)2 will precipitate Os (OH)2 (s)? K3p of Os (OH)2 i...
Questions in other subjects:


Mathematics, 14.04.2021 22:50


Mathematics, 14.04.2021 22:50

Chemistry, 14.04.2021 22:50

Mathematics, 14.04.2021 22:50



Mathematics, 14.04.2021 22:50




