
Chemistry, 06.12.2021 03:40 loganparrish2488
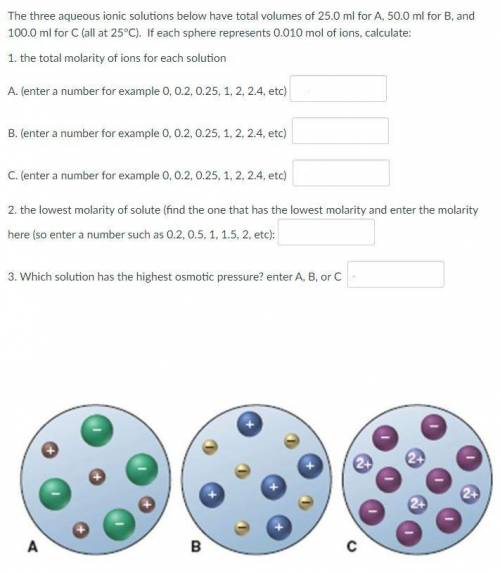
The three aqueous ionic solutions below have total volumes of 25.0 ml for A, 50.0 ml for B, and 100.0 ml for C (all at 25°C). If each sphere represents 0.010 mol of ions, calculate:
1. the total molarity of ions for each solution
2. the lowest molarity of solute
3. Which solution has the highest osmotic pressure?
See the picture attached.
My answers:
1.
A. 3.2
B. 2
C. 1.2
2. 0.4
3. A
Am I right?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, tiniecisneros28
Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn
Answers: 1

Chemistry, 22.06.2019 07:00, shradhwaip2426
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 10:40, trinityanne1738
Asolid that forms and separates from a liquid mixture is called
Answers: 2
You know the right answer?
The three aqueous ionic solutions below have total volumes of 25.0 ml for A, 50.0 ml for B, and 100....
Questions in other subjects:


Mathematics, 22.04.2020 16:48






History, 22.04.2020 16:48


Mathematics, 22.04.2020 16:49



