
Chemistry, 05.12.2021 22:30 emilypzamora11
Q1. How many moles of air are in a 2.8 x 106 L balloon at 20 °C and 750 mmHg of pressure? If the average molar mass of air is 28 g/mol, how many tons of air is this? (1 ton = 2,000 lb)?
Q2. This same balloon is heated from 20 °C to 100 °C keeping the volume and pressure constant. Calculate the new number of tons of gas inside the balloon.
Q3. Calculate the density of the air in the hot air balloon when the air inside is 100 °C in kg/m3.
(Hint: Use the mass you already calculated in question 2!)
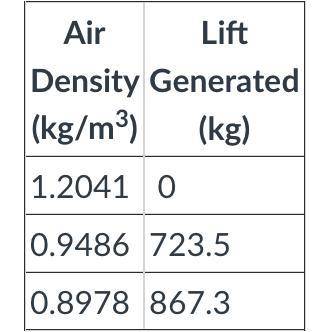
Q4. Use the table attached to determine if the balloon will float at this temperature. (Note: the total mass of the balloon and basket is 723.5 kg. You will need at least that much lift.)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, willcohen42
What postulate of the kinetic molecular theory best explains why gases have high fluidity? because collisions between gas particles are elastic, there is no loss of energy as particles flow past each other. because gases consist of large numbers of tiny particles, they spread out and do not come in contact with each other. because the attractive forces between gas particles are negligible, gas particles can glide easily past one another. because the average kinetic energy of gas particles increases as temperature increases, gas particles behave more like a liquid. question 6 compare the compressibility of gases and liquids. support your answer by describing the arrangement of particles in gases and liquids.
Answers: 1

Chemistry, 22.06.2019 02:30, carsonjohnsonn
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 11:40, Wemaybewrong
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1

Chemistry, 22.06.2019 16:00, graciewyatt6833
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
Q1. How many moles of air are in a 2.8 x 106 L balloon at 20 °C and 750 mmHg of pressure? If the ave...
Questions in other subjects:

Mathematics, 19.02.2021 14:00

Biology, 19.02.2021 14:00

Biology, 19.02.2021 14:00

Chemistry, 19.02.2021 14:00

History, 19.02.2021 14:00


Mathematics, 19.02.2021 14:00

History, 19.02.2021 14:00

Biology, 19.02.2021 14:00

History, 19.02.2021 14:00



