
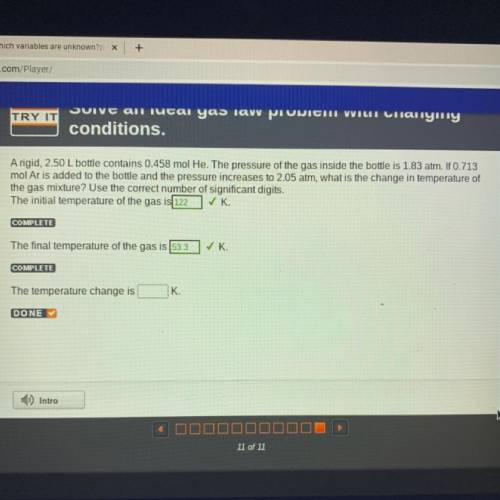
A rigid, 2.50 L bottle contains 0.458 mol He. The pressure of the gas inside the bottle is 1.83 atm. If 0.713
mol Ar is added to the bottle and the pressure increases to 2.05 atm, what is the change in temperature of
the gas mixture? Use the correct number of significant digits.
The initial temperature of the gas is 122 ✓K
COMPLETE
The final temperature of the gas is 53.3
✓ K.
COMPLETE
The temperature change is
K.
DONE

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, breannaasmith1122
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

You know the right answer?
A rigid, 2.50 L bottle contains 0.458 mol He. The pressure of the gas inside the bottle is 1.83 atm....
Questions in other subjects:

Mathematics, 25.05.2021 04:30


Spanish, 25.05.2021 04:30

Mathematics, 25.05.2021 04:30

Social Studies, 25.05.2021 04:30

Mathematics, 25.05.2021 04:30




Health, 25.05.2021 04:30



