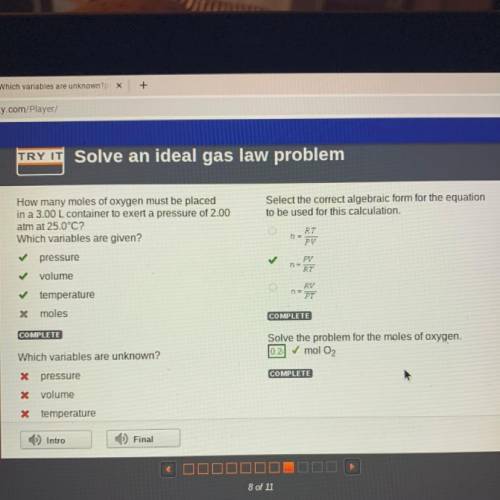
How many moles of oxygen must be placed
in a 3.00 L container to exert a pressure of 2.00
at...

Chemistry, 04.12.2021 01:30 44chandracloutier
How many moles of oxygen must be placed
in a 3.00 L container to exert a pressure of 2.00
atm at 25.0°C?
Which variables are given?
Select the correct algebraic form for the equation
to be used for this calculation.
RT
PV
pressure
✓
PV
RT
✓
volume
22
RV
PT
temperature
* moles
COMPLETE
COMPLETE
Solve the problem for the moles of oxygen.
0.2 mol O2
Which variables are unknown?
X pressure
COMPLETE
X volume
X temperature

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, cathydaves
What is the chemical formula of the following compound
Answers: 1

Chemistry, 22.06.2019 08:40, jeffcarpenter
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 11:30, chelseychew32
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 17:20, phanuel642
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2
You know the right answer?
Questions in other subjects:


Mathematics, 16.10.2020 15:01

Social Studies, 16.10.2020 15:01


Biology, 16.10.2020 15:01




Mathematics, 16.10.2020 15:01



