9.
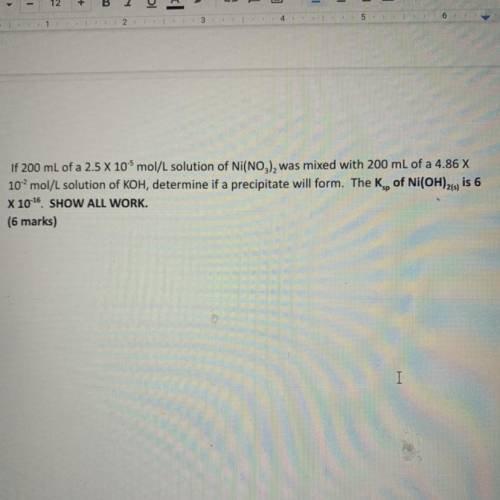
If 200 mL of a 2.5 x 10-5 mol/L solution of Ni(NO3), was mixed with 200 mL of a 4.86 X
10...

Chemistry, 02.12.2021 18:10 kstyleszdance
9.
If 200 mL of a 2.5 x 10-5 mol/L solution of Ni(NO3), was mixed with 200 mL of a 4.86 X
102 mol/L solution of KOH, determine if a precipitate will form. The Kg, of Ni(OH)21, is 6
X 10-16 SHOW ALL WORK.
(6 marks)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 23.06.2019 08:30, zhjzjzzj8225
Explain how to convert from one unit to another in the metric system.
Answers: 3

Chemistry, 23.06.2019 09:00, floressavanna15
What properties would have caused early researchers to name hydrogen "inflammable air”
Answers: 3

Chemistry, 23.06.2019 11:50, natorihill629
Charles's law describes the relationship of the volume and temperature of gas at a constant mass and pressure. according to this law, what would happen to the temperature of the gas if its volume decreased from 1.0 l to 0.50 l?
Answers: 3
You know the right answer?
Questions in other subjects:

Physics, 10.07.2019 07:00



Biology, 10.07.2019 07:00

Mathematics, 10.07.2019 07:00

Mathematics, 10.07.2019 07:00


History, 10.07.2019 07:00

Health, 10.07.2019 07:00

Mathematics, 10.07.2019 07:00



