The Mole
7
Acellus
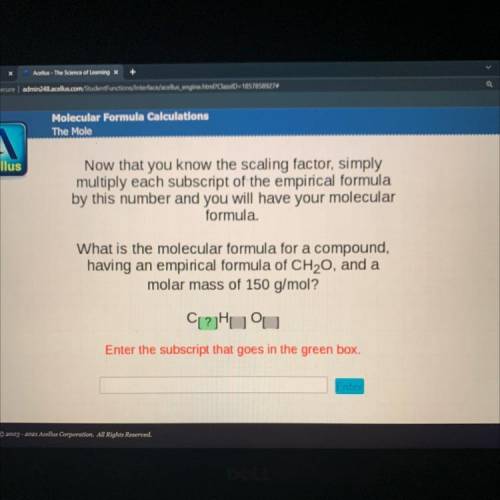
Now that you know the scaling factor, simply
multiply each su...

Chemistry, 01.12.2021 09:00 kaitlyn114433
The Mole
7
Acellus
Now that you know the scaling factor, simply
multiply each subscript of the empirical formula
by this number and you will have your molecular
formula.
on Resources
What is the molecular formula for a compound,
having an empirical formula of CH20, and a
molar mass of 150 g/mol?
CL?Huu
Enter the subscript that goes in the green box.
Enter

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3



You know the right answer?
Questions in other subjects:

Mathematics, 11.09.2020 08:01

Mathematics, 11.09.2020 08:01

Social Studies, 11.09.2020 08:01

Mathematics, 11.09.2020 08:01

Mathematics, 11.09.2020 08:01

Mathematics, 11.09.2020 08:01

English, 11.09.2020 08:01

Social Studies, 11.09.2020 08:01

Mathematics, 11.09.2020 08:01

English, 11.09.2020 08:01



