The Mole
7
Acellus
Now that you know the scaling factor, simply
multiply each su...

The Mole
7
Acellus
Now that you know the scaling factor, simply
multiply each subscript of the empirical formula
by this number and you will have your molecular
formula.
on Resources
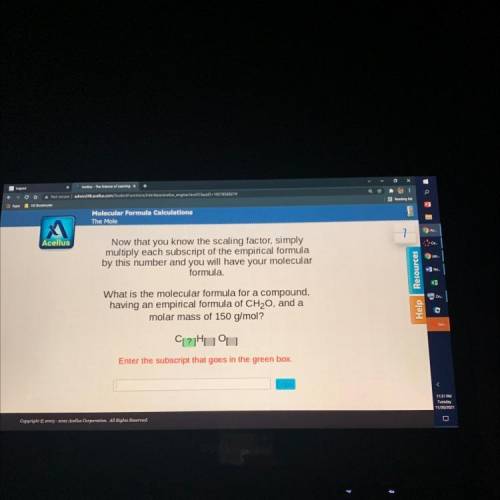
What is the molecular formula for a compound,
having an empirical formula of CH20, and a
molar mass of 150 g/mol?
CL?Huu
Enter the subscript that goes in the green box.
Enter

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, hjamya17
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1



Chemistry, 22.06.2019 21:30, leenzazou587
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
Questions in other subjects:


History, 27.10.2019 18:43

Health, 27.10.2019 18:43

Mathematics, 27.10.2019 18:43


Mathematics, 27.10.2019 18:43



History, 27.10.2019 18:43

Biology, 27.10.2019 18:43



