
Chemistry, 30.11.2021 02:10 jamesgraham577
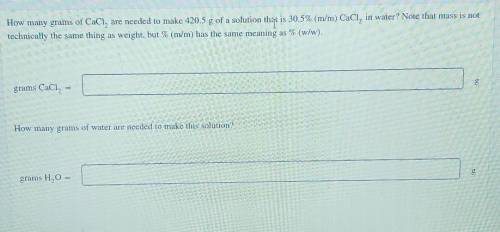
How many grams of CaCl, are needed to make 420.5 g of a solution tho is 30.5% (m/m) CaCl, in water? Note that mass is not technically the same thing as weight, but % (m/m) has the same meaning as % (w/w). grams CaCl, How many grams of water are needed to make this solution? grams H2O =

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, janetexcoelho
What does the mass of 0.7891 mol of ferric oxide (fe2o3)
Answers: 1

Chemistry, 22.06.2019 14:30, Priskittles
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 22:10, zwbaby3693
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 01:00, ZaNiyahlove4711
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
You know the right answer?
How many grams of CaCl, are needed to make 420.5 g of a solution tho is 30.5% (m/m) CaCl, in water?...
Questions in other subjects:





History, 02.09.2019 02:30


English, 02.09.2019 02:30

Mathematics, 02.09.2019 02:30

Mathematics, 02.09.2019 02:30





