Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:10, emilyplays474
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 05:30, ayoismeisjjjjuan
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

You know the right answer?
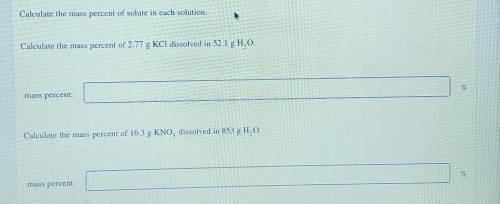
Calculate the mass percent of solute in each solution. Calculate the mass percent of 2.77 g KCl diss...
Questions in other subjects:

History, 19.08.2019 22:00





English, 19.08.2019 22:00

English, 19.08.2019 22:00


Social Studies, 19.08.2019 22:00

Chemistry, 19.08.2019 22:00