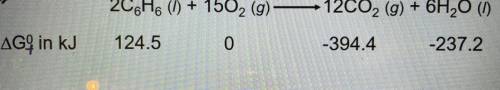Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, milesjreece3939
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 06:30, cadenhuggins2
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 08:30, Blaise2653
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3
You know the right answer?
Energyhat is the standard free-energy change for the following reaction at 25 °C?
Is the reaction...
Questions in other subjects:




Biology, 27.09.2019 12:30


Mathematics, 27.09.2019 12:30


Biology, 27.09.2019 12:30