
Chemistry, 25.11.2021 14:00 spotty2093
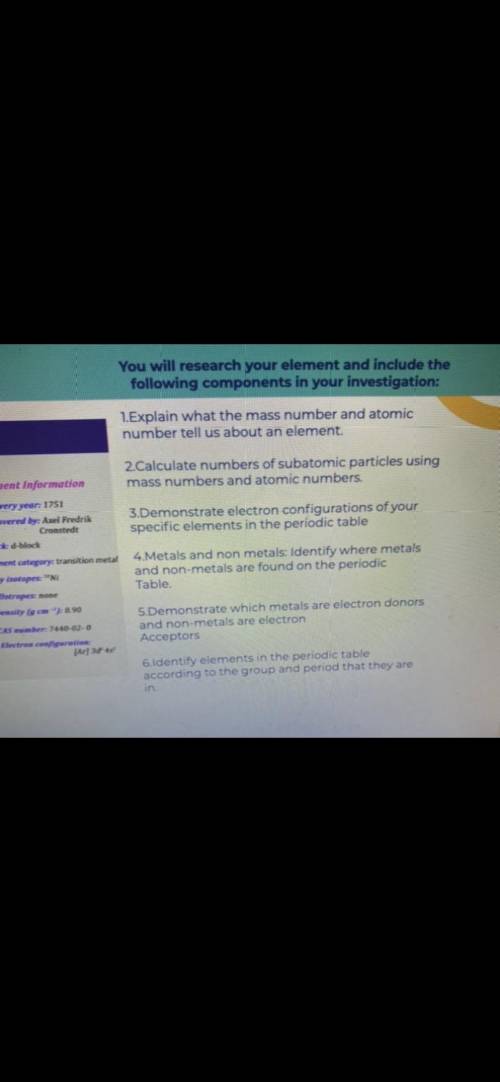
1=Explain what the mass number and atomic number tell us about an element . 2=Calculate numbers of subatomic particles using mass numbers and atomic numbers . 3=Demonstrate electron configurations of your specific elements in the periodic table 4=Metals and non metals : Identify where metals and non - metals are found on the periodic Table . 5=Demonstrate which metals are electron donors and non - metals are electron Acceptors 6=Identify elements in the periodic table according to the group and period that they are

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, sbhishop19
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 03:50, Pizzapegasus1
Express the following number in scientific notation. 0.026890 =
Answers: 1

You know the right answer?
1=Explain what the mass number and atomic number tell us about an element . 2=Calculate numbers of s...
Questions in other subjects:


Mathematics, 26.08.2020 19:01


Mathematics, 26.08.2020 19:01



English, 26.08.2020 19:01


Mathematics, 26.08.2020 19:01

Mathematics, 26.08.2020 19:01



