
Chemistry, 23.11.2021 23:00 runaway173
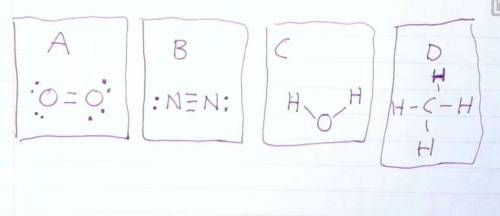
Draw the Lewis structures of N2, O2, H20, and CH4. Compare your drawing to the ones in the drawing on this test and select the answer that best describes which drawing is wrong and why.
Question 6 options:
A: O2 Is wrong because it shows the electrons at a 45 degree angle to the Oxygen atoms.
B: N2 is wrong because it shows a triple bond.
C: H2O is wrong because it is missing 4 valence electrons.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, jzjajsbdb8035
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 15:30, abdullaketbi71
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1


Chemistry, 22.06.2019 20:00, aksambo4707
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
You know the right answer?
Draw the Lewis structures of N2, O2, H20, and CH4. Compare your drawing to the ones in the drawing o...
Questions in other subjects:

Mathematics, 25.02.2020 20:42







Mathematics, 25.02.2020 20:43


Chemistry, 25.02.2020 20:43



