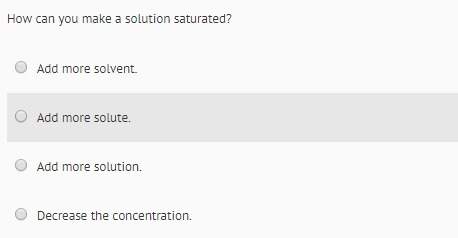
Chemistry, 23.11.2021 22:40 Boogates7427
Given the reaction Fe O2 Right arrow. Fe2(3 )O3(2-), what happens to the oxidation states of these elements? Fe O.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:10, kolbehoneyman
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion. what is the frequency f of oscillation?
Answers: 2

Chemistry, 23.06.2019 01:30, jonmorton159
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1


You know the right answer?
Given the reaction Fe O2 Right arrow. Fe2(3 )O3(2-), what happens to the oxidation states of these e...
Questions in other subjects:





Mathematics, 21.11.2019 03:31

English, 21.11.2019 03:31




English, 21.11.2019 03:31