Chemistry, 22.11.2021 23:50 sadeed00974
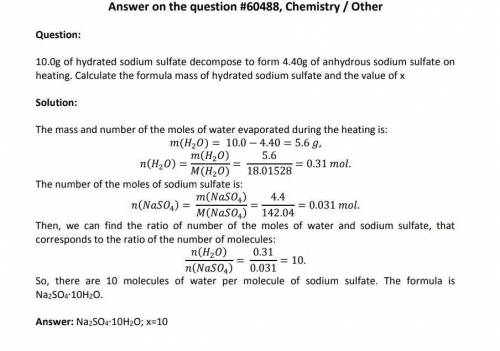
10.00g of hydrated sodium sulfate decomposes to form 4.40g Na(2)SO(4).xH(2)O -> NaSO(4) + xH(2)O of anhydrous sodium sulfate on heating. What’s the formula mass of hydrated sodium sulfate and the value of x? please help i have no clue!
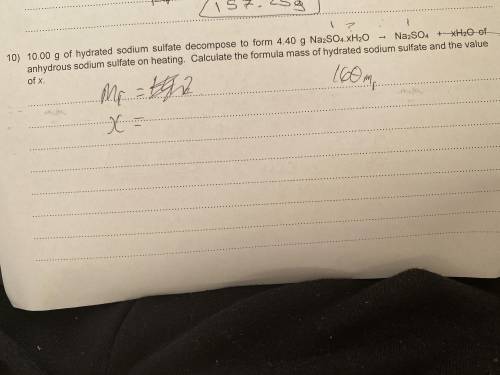

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, suemmimonjaras8374
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1
You know the right answer?
10.00g of hydrated sodium sulfate decomposes to form 4.40g Na(2)SO(4).xH(2)O -> NaSO(4) + xH(2)O...
Questions in other subjects:


Biology, 11.12.2019 18:31