
Determining Equilibrium
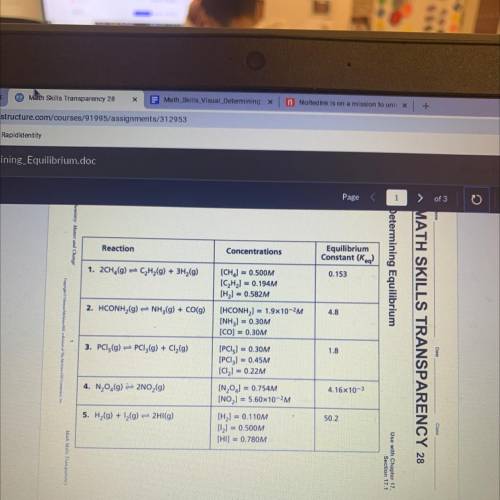
The equilibrium constants for the reactions in the table are correct at a certain
temperature. The concentrations given in the table, however, may or may not be correct
when the system is at equilibrium at that temperature. Use the information in the table to
answer the following questions.
1.On the basis of the K, values given in the table, which reaction mixture contains the largest
amount of product(s) when at equilibrium?
2. Which reaction mixture contains the largest amount of reactants when at
equilibrium? Explain.
3. Which reactions in the table have concentrations that represent the systems at
equilibrium? Explain.
4. For each reaction that is not at equilibrium, change the concentration of only one
of the reactants or products so that the ratio represents the system at equilibrium.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:20, lex68259100
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 04:30, salvadorperez26
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 17:30, nijanicole164
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
You know the right answer?
Determining Equilibrium
The equilibrium constants for the reactions in the table are correct at a...
Questions in other subjects:



Social Studies, 03.08.2019 18:30



English, 03.08.2019 18:30


English, 03.08.2019 18:30





