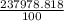Chemistry, 04.11.2021 09:30 arushiverma555
Uranium has three common isotopes: U-238 (99.2745%), U-235 (0.7200%), U-234 (0.0055%). Calculate the average atomic mass of uranium. (Round to two decimal places)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:30, xlebrny7831
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2


Chemistry, 23.06.2019 04:00, Ezekielcassese
Which method would be best to separate a mixture of sand and gravel
Answers: 1

Chemistry, 23.06.2019 05:20, cjking2320
Explain how global warming could have affected yellowstone frog and salamander habitat's, resulting in changes in the populations of these species
Answers: 2
You know the right answer?
Uranium has three common isotopes: U-238 (99.2745%), U-235 (0.7200%), U-234 (0.0055%). Calculate the...
Questions in other subjects:


Mathematics, 11.06.2020 02:57

History, 11.06.2020 02:57

Physics, 11.06.2020 02:57

Mathematics, 11.06.2020 02:57

Mathematics, 11.06.2020 02:57



Mathematics, 11.06.2020 02:57

Mathematics, 11.06.2020 02:57