
Chemistry, 03.11.2021 01:20 jabraeshaw
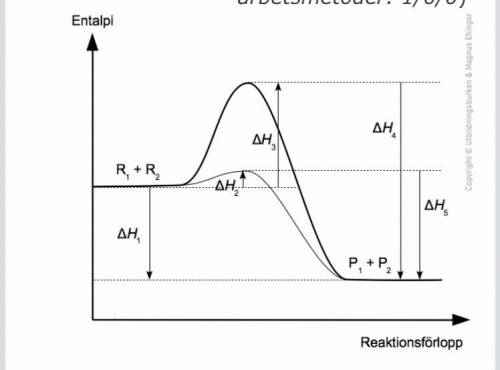
The diagram shows the course of the reaction for a chemical reaction. With and without catalyst. R stands for reactant and p stands for product. Which statement or statements are true?
1. H4 and H5 together make up the total enthalpy change of the reaction.❌?
2. The reaction is endothermic.❌
3. The presence of catalyst allows a larger amount to be formed a larger amount of the products.❌?
4. H1 <0 ✅
(EXOTERM h1 <0
(Endotherm h1> 0)
5. The activation energy is higher for the catalyzed reaction ❌?
6. The reaction is exothermic
7. H1 is the total enthalpy change ✅?
Activation energy non-catalyzed reaction = H3? ✅

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:10, Kianna000
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1

Chemistry, 22.06.2019 09:30, jewelz5887
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 22:30, arodavoarodavo
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
The diagram shows the course of the reaction for a chemical reaction. With and without catalyst. R s...
Questions in other subjects:




Biology, 23.07.2019 04:00

Mathematics, 23.07.2019 04:00

History, 23.07.2019 04:00

Physics, 23.07.2019 04:00





