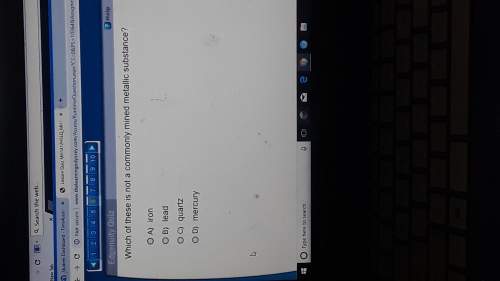
Chemistry, 30.10.2021 05:20 biancasamadp3usfw
the partial pressures of ch4, n2, and o2 in a sample of gas were found to be 143 mmhg, 469 mmhg, and 251 mmhg, respectively. calculate the mole fraction of oxygen.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, jeffcarpenter
Write a net ionic equation to show that hydrofluoric acid, behaves as an acid in water.
Answers: 1

Chemistry, 21.06.2019 22:30, pinkycupcakes3oxbqhx
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 07:00, haydjanggg6578
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2
You know the right answer?
the partial pressures of ch4, n2, and o2 in a sample of gas were found to be 143 mmhg, 469 mmhg, and...
Questions in other subjects:



Mathematics, 22.10.2020 20:01



Mathematics, 22.10.2020 20:01

Mathematics, 22.10.2020 20:01


Mathematics, 22.10.2020 20:01