A 50g block of aluminum at -100°C is submerged in 30g of water at 20°C.
Cwater = 4.184 J/gºC
...

Chemistry, 26.10.2021 19:30 lazavionadams81
A 50g block of aluminum at -100°C is submerged in 30g of water at 20°C.
Cwater = 4.184 J/gºC
AH tus water = 334 J/g
Cu = 0.89 J/gºC
What is the final temperature of both substances?
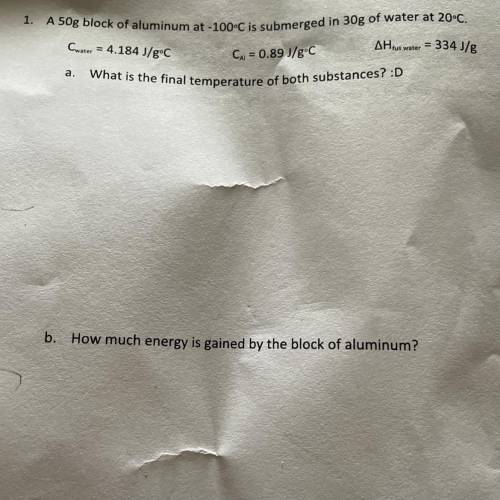

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, parisaidan366
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3


You know the right answer?
Questions in other subjects:


English, 15.12.2020 01:30

Mathematics, 15.12.2020 01:30

Chemistry, 15.12.2020 01:30


Mathematics, 15.12.2020 01:30



Mathematics, 15.12.2020 01:30




