
Chemistry, 25.10.2021 18:00 angelinaavila06
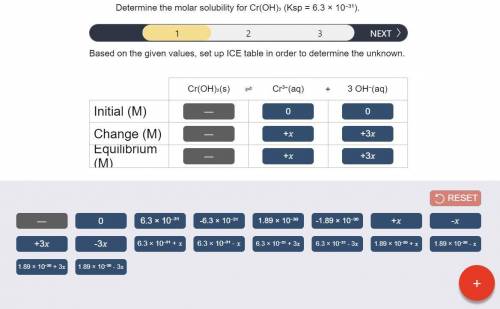
Determine the molar solubility for Cr(OH)3 Ksp = 6.3x10^-31 .
a) Set up the ICE table Cr(OH)3 (s) <-> Cr^3+ (aq) + 3OH^- (aq)
b) Ksp expression
c) Determine molar solubility



Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, gilbert325
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 14:30, CoolRahim9090
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1
You know the right answer?
Determine the molar solubility for Cr(OH)3 Ksp = 6.3x10^-31 .
a) Set up the ICE table Cr(OH)3 (s)...
Questions in other subjects:

Mathematics, 02.12.2020 06:20

Mathematics, 02.12.2020 06:20



Mathematics, 02.12.2020 06:30


Mathematics, 02.12.2020 06:30



Mathematics, 02.12.2020 06:30



