
Chemistry, 25.10.2021 01:00 ahmedeldyame
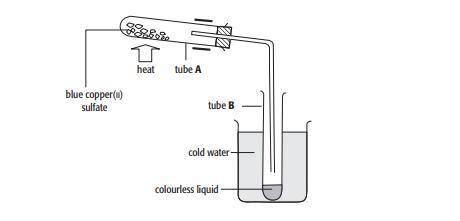
When 2.5 g of blue copper(ii) sulfate crystals were heated, 1.6 g of a white solid were left in tube A
a. Calculate the mass of water driven off in the experiment.
b. Calculate the percentage of water by mass driven off .

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, penelopymorales24
Match each object to its description: a. coma of a comet b. comet tail c. oort cloud haze surrounding a nucleus created by solar wind. hypothetical sphere around the solar system
Answers: 1

Chemistry, 22.06.2019 05:40, yah2muchh
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 08:30, Apple557
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1
You know the right answer?
When 2.5 g of blue copper(ii) sulfate crystals were heated, 1.6 g of a white solid were left in tube...
Questions in other subjects:


Mathematics, 11.11.2019 11:31

History, 11.11.2019 11:31



English, 11.11.2019 11:31



English, 11.11.2019 11:31

Biology, 11.11.2019 11:31



