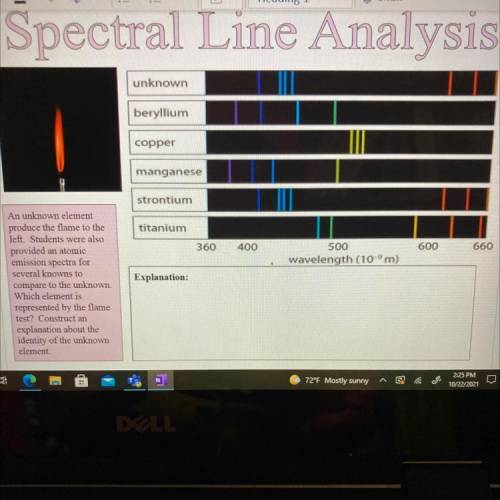
An unknown element
produce the flame to the
left. Students were also
provided an atomi...

Chemistry, 24.10.2021 08:20 jennelledenise
An unknown element
produce the flame to the
left. Students were also
provided an atomic
emission spectra for
several knowns to
compare to the unknown.
Which element is
represented by the flame
test? Construct an
explanation about the
identity of the unknown
element.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 14:00, JJlover1892
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2
You know the right answer?
Questions in other subjects:









Chemistry, 06.10.2019 03:00

Biology, 06.10.2019 03:00



