
Chemistry, 23.10.2021 20:50 raquelrivera03
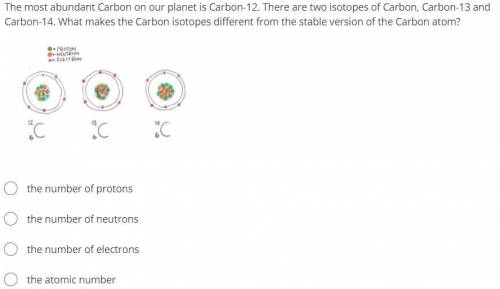
The most abundant Carbon on our planet is Carbon-12. There are two isotopes of Carbon, Carbon-13 and Carbon-14. What makes the Carbon isotopes different from the stable version of the Carbon atom?
the number of protons
the number of neutrons
the number of electrons
the atomic number

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, nikkih1225
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 08:30, melikefood01
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

You know the right answer?
The most abundant Carbon on our planet is Carbon-12. There are two isotopes of Carbon, Carbon-13 and...
Questions in other subjects:



Mathematics, 25.09.2021 22:20

Biology, 25.09.2021 22:20

History, 25.09.2021 22:20


Mathematics, 25.09.2021 22:20


Mathematics, 25.09.2021 22:20



