
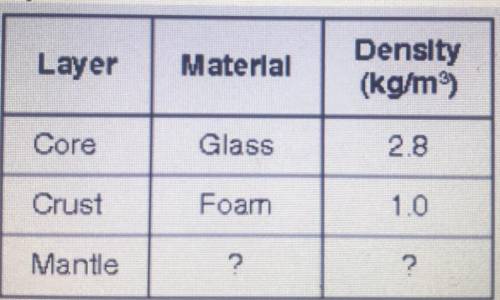
A student is making a model of the layers of Earth. She is using materials with different densities to represent the layers. The table shows the materials she has chosen for two layers.
How should the density of the material the student uses for the mantle compare to the density of the materials chosen for the other layers?
It should be more dense than foam.
It should be more dense than glass,
It should be the same density as foam
It should be the same density as glass.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:10, yootmytoot
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution. calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 18:30, bibiansolis
The table lists the lattice energies of some compounds. compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf. the lattice energy increases as the cations get larger, as shown by lif and licl. the lattice energy decreases as cations get smaller, as shown by nacl and naf. the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 22.06.2019 22:20, trockout4868
How do cfcs cause ozone depletion? how do cfcs cause ozone depletion? ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule.
Answers: 2
You know the right answer?
A student is making a model of the layers of Earth. She is using materials with different densities...
Questions in other subjects:

Mathematics, 20.01.2021 05:00


Mathematics, 20.01.2021 05:00

Business, 20.01.2021 05:00

English, 20.01.2021 05:00

Chemistry, 20.01.2021 05:00


Mathematics, 20.01.2021 05:00

Mathematics, 20.01.2021 05:00

Mathematics, 20.01.2021 05:00



