
Chemistry, 18.10.2021 08:30 untouchedyannaa
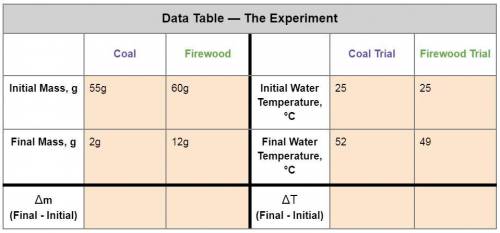
1. Calculate the heat gained by the water when the Firewood was burned.
Equation: q=mc(T f-Ti)
q = heat (J)
m = mass of the water = 50g
c is a constant = 4.184
T f = final temperature
Ti = initial temperature
-
2. Calculate the heat gained by the water when the Coal was burned.
Equation: q=mc(T f-Ti)
q = heat (J)
m = mass of the water = 50g
c is a constant = 4.184
T f = final temperature
Ti = initial temperature

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, VictoriaRose520
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 12:30, Svetakotok
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 23.06.2019 00:30, StayPuftMarshadowMan
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1

Chemistry, 23.06.2019 09:00, blossie94681
The vapor pressure of water at 25.0°c is 23.8 torr. determine the mass of glucose (molar mass = 180 g/mol) needed to add to 500.0 g of water to change the vapor pressure to 22.8 torr.
Answers: 1
You know the right answer?
1. Calculate the heat gained by the water when the Firewood was burned.
Equation: q=mc(T f-Ti)
Questions in other subjects:

Mathematics, 07.10.2020 23:01





English, 07.10.2020 23:01


Mathematics, 07.10.2020 23:01


Mathematics, 07.10.2020 23:01



