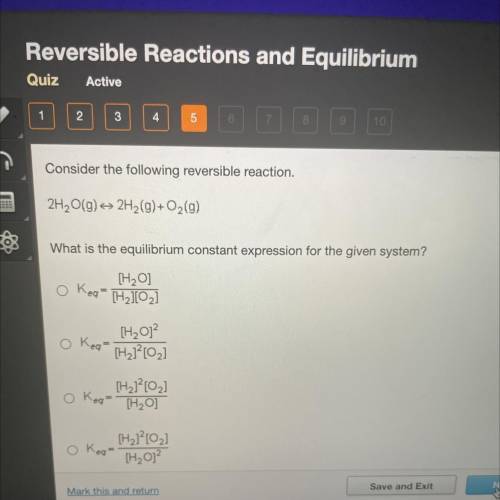
Consider the following reversible reaction.
2H2O(g) + 2H2(9)+O2(g)
...

Chemistry, 17.10.2021 14:30 nessross1018
Consider the following reversible reaction.
2H2O(g) + 2H2(9)+O2(g)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, sammiehammer
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2


Chemistry, 22.06.2019 09:50, revlonknox6
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 21:30, djdjdjdbdbjx
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
Questions in other subjects:


Biology, 13.12.2019 18:31

Mathematics, 13.12.2019 18:31



English, 13.12.2019 18:31


Mathematics, 13.12.2019 18:31

Mathematics, 13.12.2019 18:31




