
Chemistry, 14.10.2021 01:00 abelinoperez652
if 1.40 mol mg are mixed with 2.00 mol of HCl, how many moles of excess reactant remain after the reaction is complete?
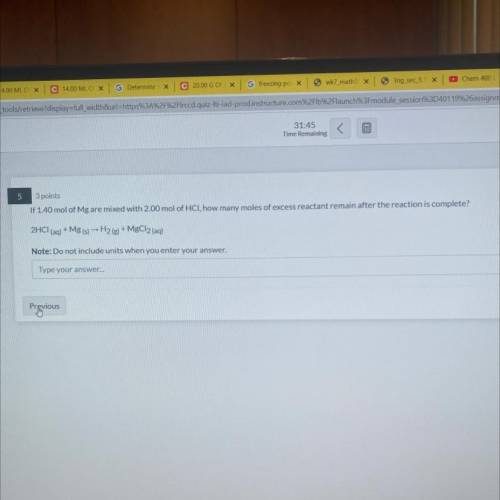

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, thatonestudent2271
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 02:30, caeyanij
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 07:50, mckinleesmomp6qj1e
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 12:30, quantamagic
Word equation for k(s)+h2o(l) yield koh(aq) + h2(g)
Answers: 1
You know the right answer?
if 1.40 mol mg are mixed with 2.00 mol of HCl, how many moles of excess reactant remain after the re...
Questions in other subjects:




Computers and Technology, 06.10.2019 04:30

Mathematics, 06.10.2019 04:30

History, 06.10.2019 04:30




Mathematics, 06.10.2019 04:30



