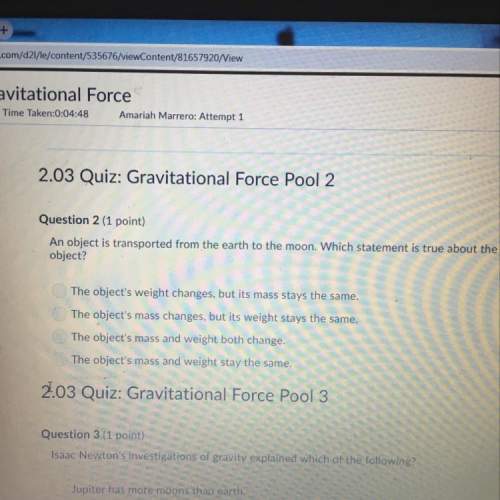
Chemistry, 11.10.2021 21:20 chloelandry
A galvanic cell consists of a chromium, Cr bar in a chromic sulfate solution, Cr2(SO4)3 containing 13 g and a silver, Ag bar in a silver sulfate solution (Ag2SO4 ) containing 37.8 g in a
3 L solution and a sodium sulfate solution use as a salt bridge solution. The silver electrode is positive relative to the chromium electrode. Write the cell half- reactions, overall reaction and cell notation. Calculate the following:
A) Gibbs Free – Energy B) Electrode potential of the cell.
(Note: when calculating the molarity do not include the spectator ions)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, tbeck225
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 08:00, wizz4865
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

You know the right answer?
A galvanic cell consists of a chromium, Cr bar in a chromic sulfate solution, Cr2(SO4)3 containing 1...
Questions in other subjects:




Mathematics, 20.03.2020 11:22