
Chemistry, 11.10.2021 18:40 donnafranks2003
An excess of sodium carbonate, Na, CO3, in solution is added to a solution containing 17.87 g CaCl2. After performing the experiment, 13.19 g of calcium carbonate, CaCO3, is produced. Calculate the percent yield of this reaction.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 04:31, laurenbreellamerritt
How big are the bighest ocean waves at mavericks
Answers: 1

Chemistry, 23.06.2019 15:40, bignutz29
The poh of a solution is 6.0. which statement is correct? use poh=-log[oh and ph+poh= 14 o o o the ph of the solution is 20.0. the concentration of oh ions is 1.0 x 10-8 m- the concentration of oh ions is 1.0 x 106 m- the ph of the solution is 8.0.
Answers: 3
You know the right answer?
An excess of sodium carbonate, Na, CO3, in solution is added to a solution containing 17.87 g CaCl2....
Questions in other subjects:

Biology, 03.07.2019 21:00

Mathematics, 03.07.2019 21:00

Mathematics, 03.07.2019 21:00

Mathematics, 03.07.2019 21:00

English, 03.07.2019 21:00

English, 03.07.2019 21:00

History, 03.07.2019 21:00



English, 03.07.2019 21:00

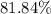 .
.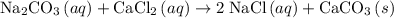 .
.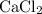 , as well as those in the product of interest,
, as well as those in the product of interest, 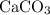 :
: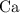 :
: 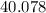 .
.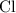 :
: 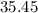 .
. :
: 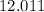 .
. :
:  .
.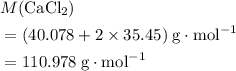 .
.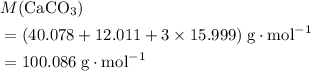 .
.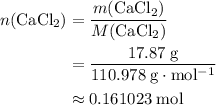 .
.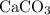 ) are both
) are both  . Thus:
. Thus: .
. 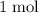 of
of 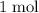 of
of 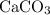 in this experiment:
in this experiment: .
. .
. , calculate the percentage yield of this experiment:
, calculate the percentage yield of this experiment: .
.

