
Chemistry, 06.10.2021 22:20 tatertottheyoungin
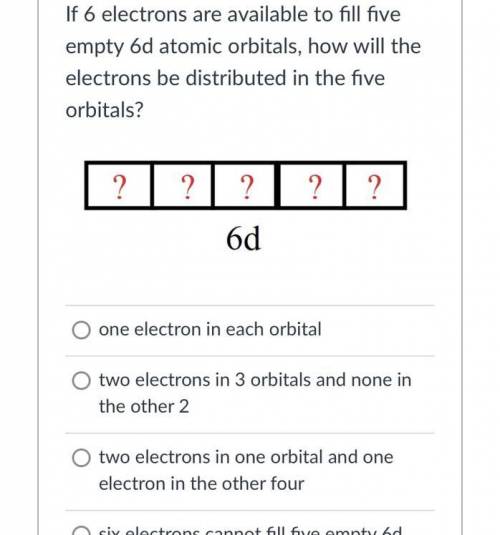
If 6 electrons are available to fill fiveempty 6d atomic orbitals, how will theelectrons be distributed in the fiveorbitals?? ? ? 2 ?6dO one electron in each orbitalO two electrons in 3 orbitals and none inthe other 2two electrons in one orbital and oneelectron in the other fourO six electrons cannot fill five empty 6datomic orbitals

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:40, markipler01
What is the study of how matter and energy interact? a. biology b. physics c. planetary science d. chemistry
Answers: 1

Chemistry, 22.06.2019 05:30, greekfreekisdbz
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 10:30, tjjjjjjjjjjjjjjjjjjj
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1
You know the right answer?
If 6 electrons are available to fill fiveempty 6d atomic orbitals, how will theelectrons be distribu...
Questions in other subjects:

Biology, 09.12.2020 15:50

English, 09.12.2020 15:50



Social Studies, 09.12.2020 15:50


Biology, 09.12.2020 15:50

Engineering, 09.12.2020 15:50


Biology, 09.12.2020 15:50



