
Chemistry, 06.10.2021 21:20 davidswafforddd478
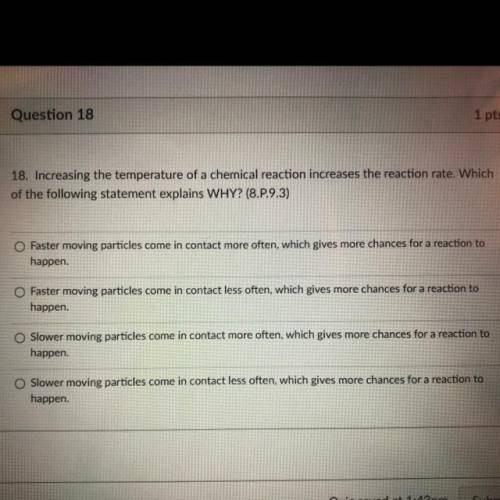
18. Increasing the temperature of a chemical reaction increases the reaction rate. Which
of the following statement explains WHY? (8.P.9.3)
O Faster moving particles come in contact more often, which gives more chances for a reaction to
happen.
Faster moving particles come in contact less often, which gives more chances for a reaction to
happen
Slower moving particles come in contact more often, which gives more chances for a reaction to
happen.
Slower moving particles come in contact less often, which gives more chances for a reaction to
happen.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 03:00, winterblanco
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3


Chemistry, 23.06.2019 11:30, cjmckee2001
How do you calculate the mass of a product when the amounts of more than one reactant are given?
Answers: 3
You know the right answer?
18. Increasing the temperature of a chemical reaction increases the reaction rate. Which
of the fo...
Questions in other subjects:




Mathematics, 10.06.2020 05:57



Mathematics, 10.06.2020 05:57


English, 10.06.2020 05:57



