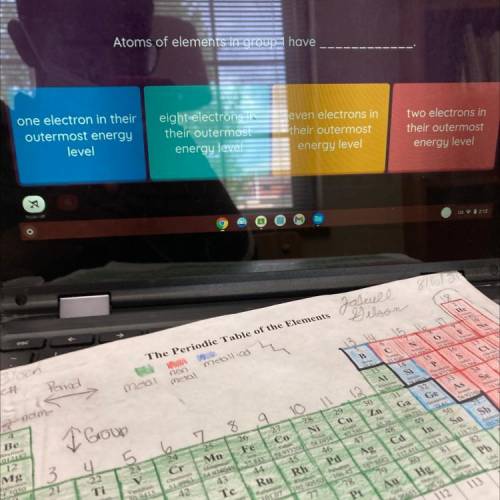
Atoms of elements in group 1 have
one electron in their
outermost energy
level
e...

Chemistry, 05.10.2021 14:00 martintrhernandez
Atoms of elements in group 1 have
one electron in their
outermost energy
level
eight electrons in
their outermost
energy level
seven electrons in
their outermost
energy level
two electrons in
their outermost
energy level

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:10, dookiefadep5n1tt
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1


Chemistry, 23.06.2019 00:30, rose888829
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2
You know the right answer?
Questions in other subjects:

English, 16.01.2020 16:31



Biology, 16.01.2020 16:31

History, 16.01.2020 16:31

Mathematics, 16.01.2020 16:31

World Languages, 16.01.2020 16:31

History, 16.01.2020 16:31




