
Chemistry, 04.10.2021 14:00 daniel1480
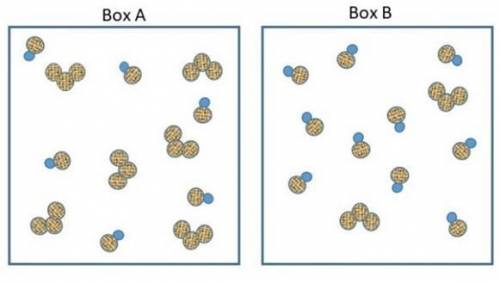
The two pictures shown below represent starting conditions for the following reaction: O3 (g) + NO(g) → O2 (g) + NO2 (g) with a rate law: Rate = k (O3 )(NO)
Which flask will react faster than the other? Determine how much faster the fast one reacts compared to the slow one? Explain your answers in terms of molecular collisions.
How do I find the answer to this? My thinking is that box a will react more since there's equal amounts of reactants but box b will react faster since there's so much NO to collide with the O3 molecules. Thanks.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, zaehairston78531
What is the nature of the ca-cl bond in a molecule of calcium chloride (cacl2) if the electronegativity value of calcium is 1.0 and that of chlorine is 3.16?
Answers: 1

Chemistry, 21.06.2019 20:30, johngayden46
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2


Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1
You know the right answer?
The two pictures shown below represent starting conditions for the following reaction: O3 (g) + NO(g...
Questions in other subjects:





Mathematics, 04.02.2021 19:30

Mathematics, 04.02.2021 19:30


History, 04.02.2021 19:30

Mathematics, 04.02.2021 19:30



