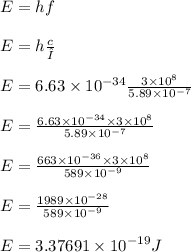Calculate the energy of one photon of yellow light that has a wavelength of 5.89x10^-7m.
...

Chemistry, 04.10.2021 08:40 Lilabsterdoll
Calculate the energy of one photon of yellow light that has a wavelength of 5.89x10^-7m.


Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, ethanmel21
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2



Chemistry, 22.06.2019 11:00, RidhaH
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural. question 2 reflects a moral or social value. question 3 refers to something that can be measured. question 4 reflects a question that can’t be observed.
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 22.10.2020 17:01

Biology, 22.10.2020 17:01

History, 22.10.2020 17:01

Mathematics, 22.10.2020 17:01

Mathematics, 22.10.2020 17:01




Mathematics, 22.10.2020 17:01