Chemistry, 03.10.2021 17:40 haileysolis5
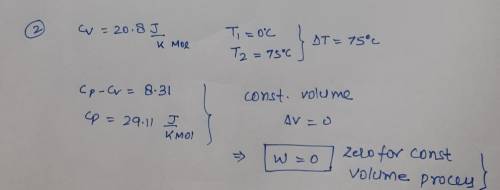
One mole of an ideal gas, with Cv = 20.8 JK-Imol-1 is transformed at
constant volume from 0 °C to 75 °C. Calculate q, w, AU, and AH for
this process. Note that 1 L atm = 101.325 J.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:30, simihehe
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 22.06.2019 20:30, lexibyrd120
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2
You know the right answer?
One mole of an ideal gas, with Cv = 20.8 JK-Imol-1 is transformed at
constant volume from 0 °C to...
Questions in other subjects:


History, 15.10.2020 20:01

Physics, 15.10.2020 20:01


Mathematics, 15.10.2020 20:01



History, 15.10.2020 20:01


Mathematics, 15.10.2020 20:01