
Chemistry, 03.10.2021 01:00 memoryofdale
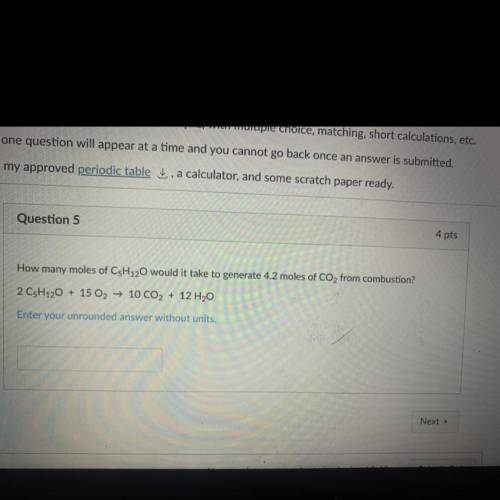
How many moles of C5H120 would it take to generate 4.2 moles of CO2 from combustion?
2 CsH120 + 15 O2 → 10 CO2 + 12 H2O
Enter your unrounded answer without units.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, Makoshark6887
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1


Chemistry, 22.06.2019 22:40, lindseyklewis1p56uvi
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization. a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution. part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 23.06.2019 04:40, yayamcneal05
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1
You know the right answer?
How many moles of C5H120 would it take to generate 4.2 moles of CO2 from combustion?
2 CsH120 + 15...
Questions in other subjects:



Mathematics, 07.05.2020 00:13


Mathematics, 07.05.2020 00:13

History, 07.05.2020 00:13

English, 07.05.2020 00:13



Mathematics, 07.05.2020 00:13



