1 point
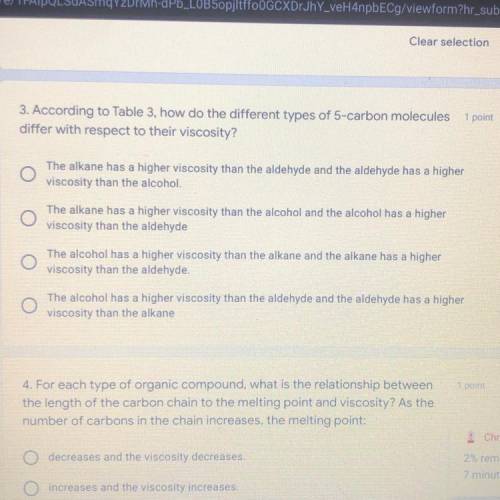
3. According to Table 3, how do the different types of 5-carbon molecules
differ wit...

Chemistry, 29.09.2021 19:00 esmemaluma00
1 point
3. According to Table 3, how do the different types of 5-carbon molecules
differ with respect to their viscosity?
The alkane has a higher viscosity than the aldehyde and the aldehyde has a higher
viscosity than the alcohol.
The alkane has a higher viscosity than the alcohol and the alcohol has a higher
viscosity than the aldehyde
The alcohol has a higher viscosity than the alkane and the alkane has a higher
viscosity than the aldehyde.
The alcohol has a higher viscosity than the aldehyde and the aldehyde has a higher
viscosity than the alkane

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, hala201490
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1



Chemistry, 23.06.2019 03:30, tamariarodrigiez
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 07.09.2020 03:01








English, 07.09.2020 03:01



