
Chemistry, 29.09.2021 02:20 ptanner706
Determine the rate of the reaction shown directly below if the rate constant k is 1.1 x 10^–2 M^–2 s^–1, the NO concentration is 5.0 x 10^–4 M, and the H2 concentration of 8.0 x 10^–2 M Thank you! :)
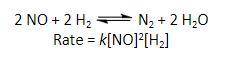

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, bettybales1986
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 19:00, Jasoncookies23
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

Chemistry, 22.06.2019 19:20, evansh78
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
Determine the rate of the reaction shown directly below if the rate constant k is 1.1 x 10^–2 M^–2 s...
Questions in other subjects:






English, 03.12.2019 19:31






