
Chemistry, 27.09.2021 14:00 xscape0mari
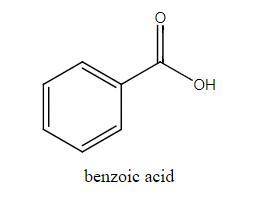
The molar mass of benzoic acid (C6H5COOH) determined by measuring the freezing‐point depression in benzene is twice what we would expect for the molecular formula, C7H6O2. Explain this apparent anomaly. Thank you! :)

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 06:10, tammydbrooks43
How much would the freezing point of water decrease if 4 mol of nacl were added to 1 kg of water (kf=1.86 degrees c/(mol/kg) for water and i=2 for nacl a- 7.44 degrees c b- 14.88 c 3.72 d 1.86
Answers: 1

Chemistry, 23.06.2019 07:30, fernandancon1872
Assume that 13.5 g solid aluminum (al) react with hcl to produce solid aluminum chloride (alcl3) salt and gaseous hydrogen (h2) at standard temperature and pressure.
Answers: 1
You know the right answer?
The molar mass of benzoic acid (C6H5COOH) determined by measuring the freezing‐point depression in b...
Questions in other subjects:












