
Chemistry, 27.09.2021 01:00 mrsdeanwinchester18
Cho dung dịch Ba(OH)2 dư vào 50ml dung dịch X chứa các ion NH4+; SO42-; NO3- đun nóng thì có 11,65g kết tủa xuất hiện và có 4,48lít khí Y thoát ra. Nồng độ mỗi muối trong dung dịch X là
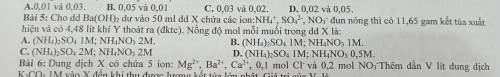

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:40, taysomoneyyy
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2


Chemistry, 23.06.2019 03:00, duplessistoccara
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 2
You know the right answer?
Cho dung dịch Ba(OH)2 dư vào 50ml dung dịch X chứa các ion NH4+; SO42-; NO3- đun nóng thì có 11,65g...
Questions in other subjects:




Mathematics, 01.09.2021 02:20

Mathematics, 01.09.2021 02:20

Mathematics, 01.09.2021 02:20

Mathematics, 01.09.2021 02:20

Mathematics, 01.09.2021 02:20


History, 01.09.2021 02:20



